| DIETHYLENE GLYCOL DIACETATE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 628-68-2 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 211-049-4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FORMULA | C8H14O5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. | 190.20 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| H.S. CODE | 2915.39 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | 2,2'-Oxybisethanol, Diacetate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3-Oxapentane-1,5-diyl diacetate; Oxydiethylene acetate; Diglycol, diacetate; 2-[2-(Acetyloxy)ethoxy]ethyl acetate; Oxydiethylendi(acetat); Di(acetato) de oxidietileno (Spanish}; Di(acétate) d'oxydiéthylène (French); | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
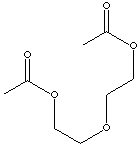
SMILES |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | clear liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | 18 - 20 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY |
1.115 - 1.120 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS | Health: 1; Flammability: 2; Reactivity: 0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| REFRACTIVE INDEX |
1.430 - 1.432 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT | 135 C | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY |
Stable under ordinary conditions |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Glycol: any of a
class of organic chemicals characterized by having separate two hydroxyl (-OH)
groups, contribute to high water solubility, hygroscopicity and reactivity with
many organic compounds, on usually linear and aliphatic carbon chain. The
general formula is CnH2n(OH)2 or
(CH2)n(OH)2. The wider meaning
names include diols, dihydric alcohols, and dihydroxy alcohols. Polyethylene
glycols and polypropylene glycols are sometimes called polyglycols which are
derived by polymerization of ethylene oxide and propylene oxide respectively.
Polyethylene glycols are water-soluble at all molecular weights, but
polypropylene glycols become increasingly less water-soluble at high molecular
weights. Ethylene glycol, HOCH2CH2OH, is the simplest
member of the glycol family. Mono-, di- and triethylene glycols are the first
three members of a homologous series of dihydroxy alcohols. They are colourless,
essentially odourless stable liquids with low viscosities and high boiling
points. Ethylene glycol is a colourless, odourless, involatile and hygroscopic
liquid with a sweet taste. It is somewhat viscous liquid; miscible with water;
boiling point 198 C, melting point 13 C; soluble in ethanol, acetone, acetic
acid, glycerine, pyridine, aldehydes; slightly soluble in ether; insoluble in
oil, fat, hydrocarbones. It is prepared commercially by oxidation of ethylene at
high temperature in the presence of silver oxide catalyst, followed by hydration
of ethylene oxide to yield mono-, with di-, tri-, and tetraethylene glycols as
co-products. The yields of ethylene glycol are depend on pH conditions. The
acid-catalyzed condition in the presence of excess water provides the highest
yield of monoethylene glycol. Because of its low freezing point, involatility
and low corrosive activity, it is widely used in mixtures of automobile
antifreeze and engine-cooling liquids. Ethylene glycol has become increasingly
important in the plastics industry for the manufacture of polyester fibers and
resins, including polyethylene terephthalate, which is used to make plastic
bottles for soft drinks (PET bottles). MEG is the raw material in the production
of polyester fiber, PET resins, alkyd, and unsaturated polyester. Diethylene
glycol, CH2OHCH2OCH2CH2OH, is similar in
properties to MEG, but with a higher boiling point, viscosity, and specific
gravity. Diethylene glycol is used in the manufacture of unsaturated polyester
resins, polyurethanes and plasticizers. It is a water-soluble liquid; boiling
point 245 C; soluble in many organic solvents. It is used as a humectant in the
tobacco industry and in the treatment of corks, glue, paper and cellophane.
Diethylene glycol (DEG) is derived as a co-product with ethylene glycol and
triethylene glycol. The industry generally operates to maximize MEG production.
Ethylene glycol is by far the largest volume of the glycol products in a variety
of applications. Availability of DEG will depend on demand for derivatives of
the primary product, ethylene glycol, rather than on DEG market requirements.
Triethylene glycol, HO(C2H4O)3H, is a colourless,
odourless, non-volatile, and hygroscopic liquid. It is characterised by two
hydroxyl groups along with two ether linkages, which contribute to its high
water solubility, hygroscopicity, solvent properties and reactivity with many
organic compounds. DEG is used in the synthesis of morpholine and 1,4-dioxane.
TEG is displacing diethylene glycol in many of these applications on account of
its lower toxicity. TEG finds use as a vinyl plasticizer, as an intermediate in
the manufacture of polyester resins and polyols, and as a solvent in many
miscellaneous applications. Triethylene glycol (TEG) is derived as a coproduct
in the manufacture of ethylene glycol from ethylene oxide, and from "on-purpose"
TEG production using diethylene glycol. Some capacities are based on total
capacity for ethylene glycols. The main uses for TEG depend upon its hygroscopic
properties. Air conditioning systems use TEG as dehumidifiers and, when
volatilized, as an air disinfectant for bacteria and virus control. Glycols,
having high boiling point and affinity for water, are employed as liquid
desiccant for the dehydration of natural gas. The dehydration means the removal
of water vapor in refinery tower so that dry hydrocarbon gases can exit from the
top of the tower. There are wide range of glycol ethers which have bifunctional
nature of ether and alcohol. cellosolves are monoether derivatives of ethylene
glycol. They are excellent solvents, having solvent properties of both ethers
and alcohols. Glycol family products are versatile compounds used in the fields
include;
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
clear liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ESTER CONTENT |
94.0% min |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
WATER |
0.2% max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
COLOR, APHA |
50 max |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING | 200kgs in drum | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| OTHER INFORMATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Symbols: F, Risk Phrases: 45-46-60-23/24/25-36/37/38-48/23/24/25-63, Safety Phrases: 53-23-36/37/39-45 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||